Neat Tips About How To Increase The Rate Of A Chemical Reaction

Download rate of reaction cheat sheet by clicking on the button below.
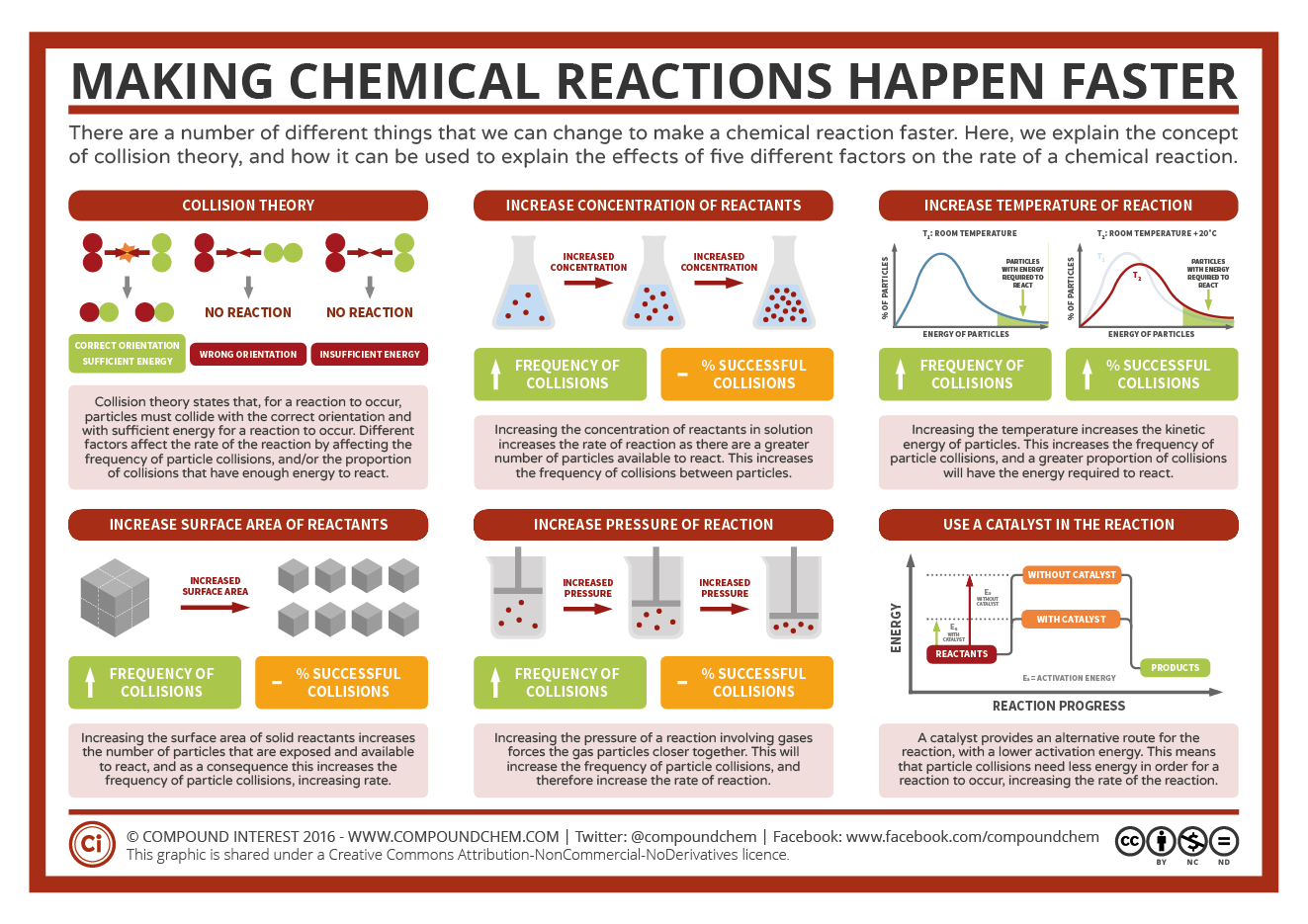
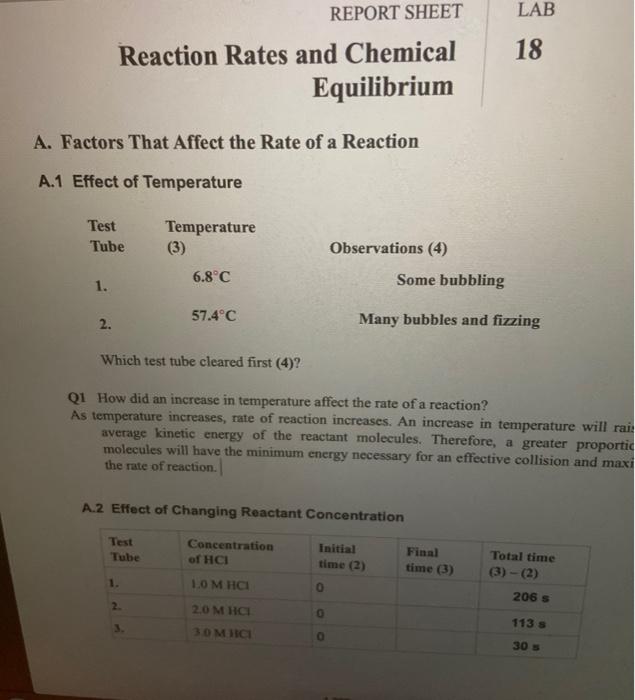
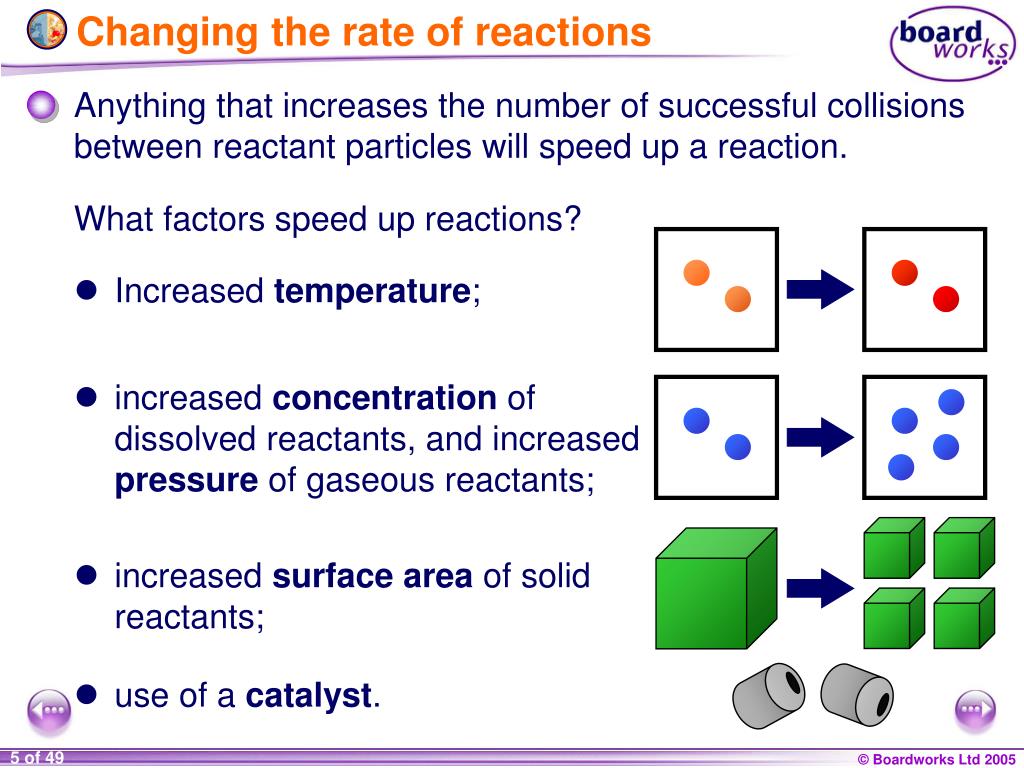
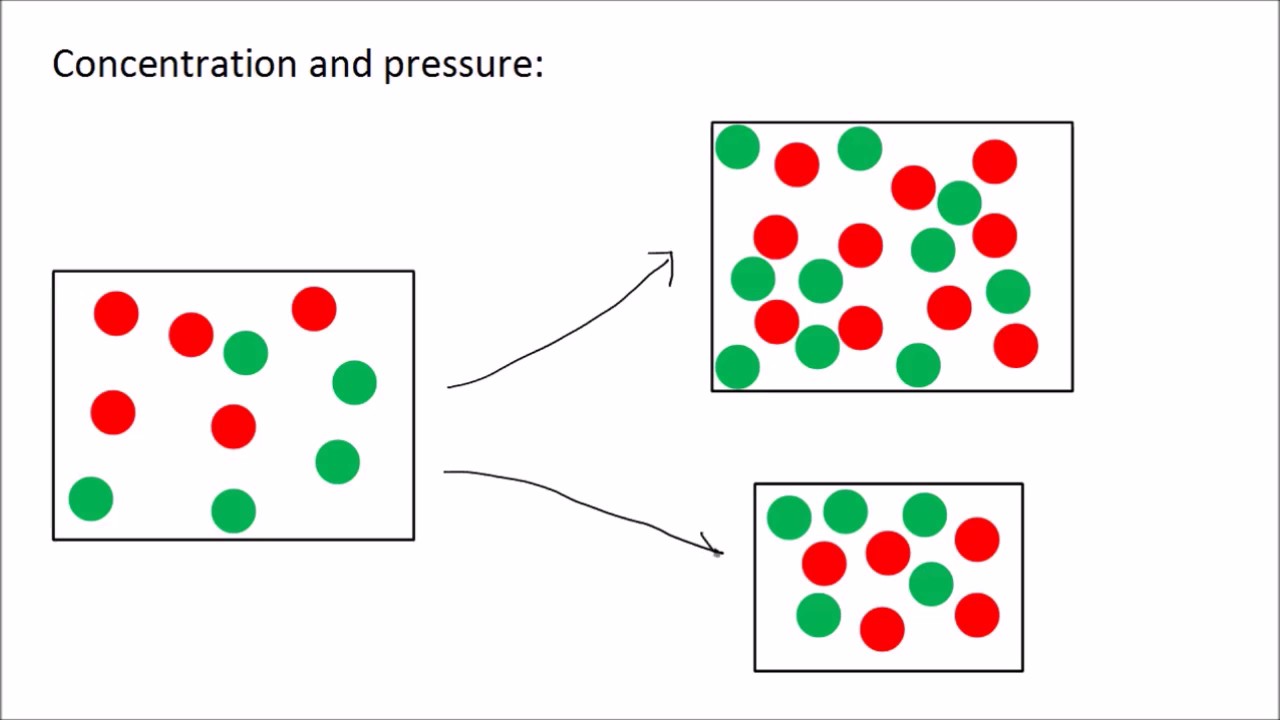
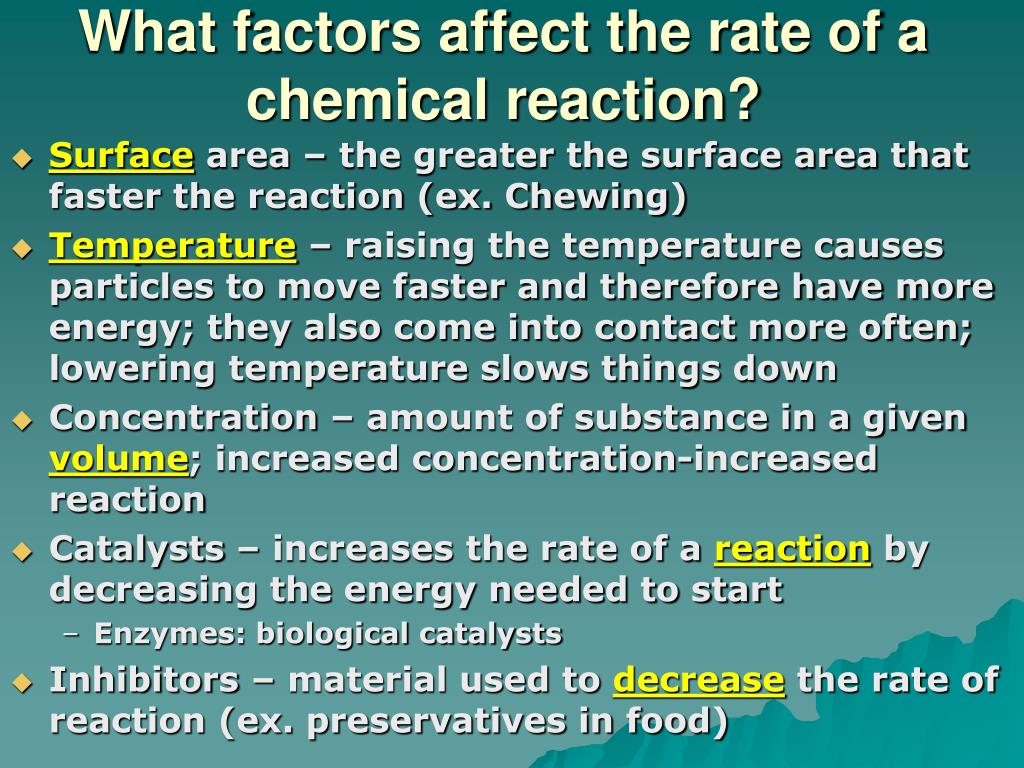
How to increase the rate of a chemical reaction. Pressure factor pressure increases the concentration of gases which in turn results in the increase of the rate of reaction. It is important that chemists can control the rate of chemical reactions to ensure that processes are both economically viable (they will result in a good yield of products and. Usually reactions speed up with increasing temperature.
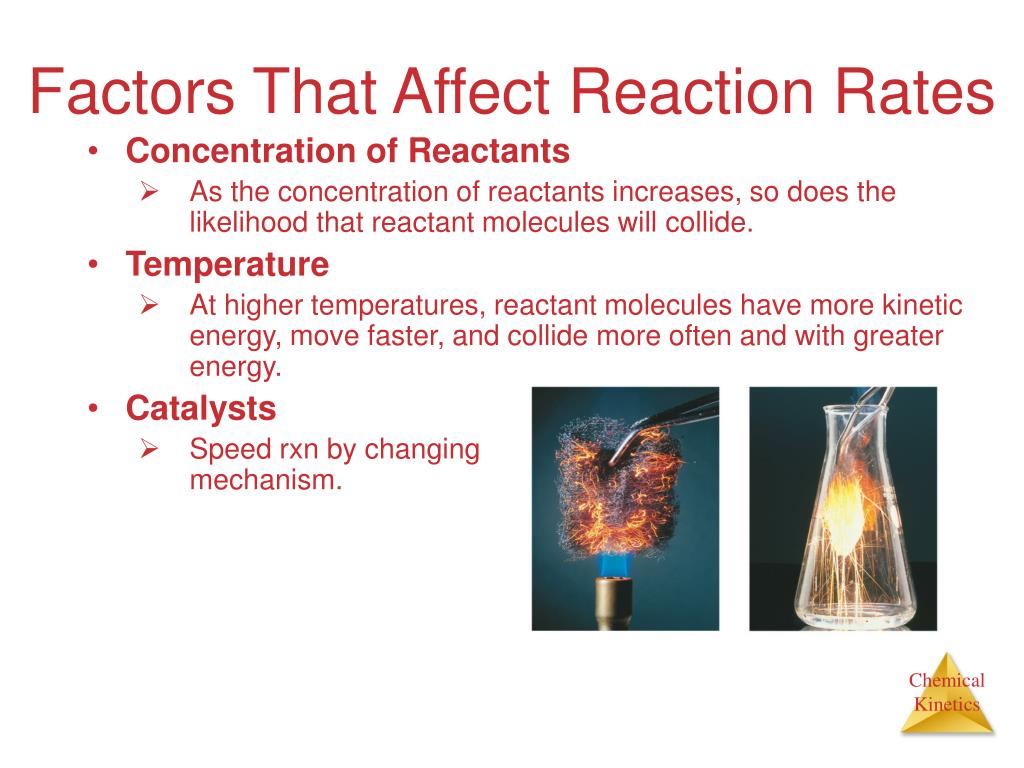
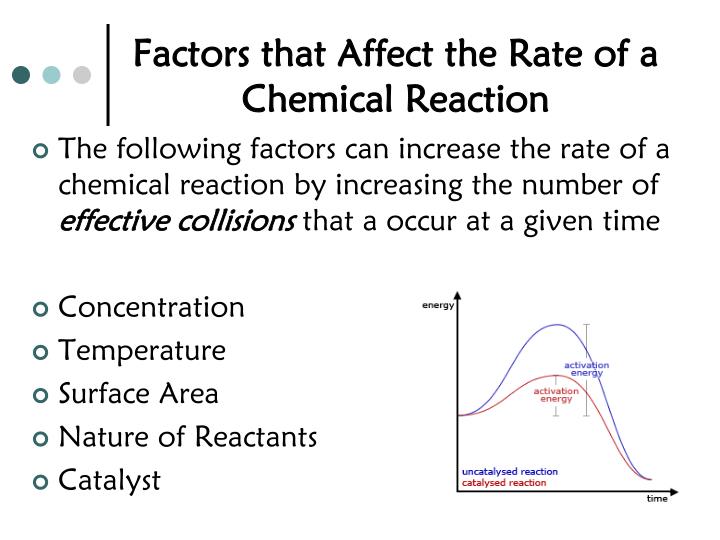
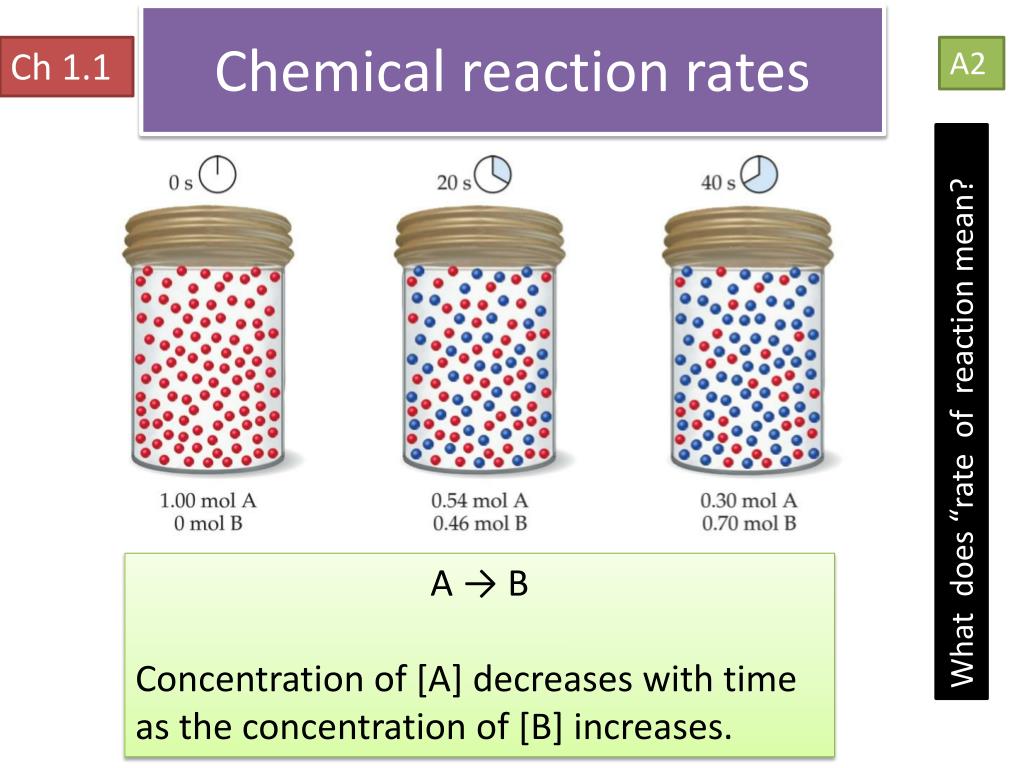
The reaction rate with a large \(e_a\) increases rapidly with increasing temperature, whereas the reaction rate with a smaller \(e_a\) increases much more. The measure of the change in the concentration of the reactants or products per time unit is referred to as the reaction rate for a particular chemical reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction.
The equal mass of sugar. Reaction mechanisms reaction mechanism and rate law google classroom key points a reaction mechanism is the sequence of elementary steps by which a chemical reaction. Rate of a chemical reaction computed as the ratio of a measured change in amount or concentration of substance to the time interval over which the change.
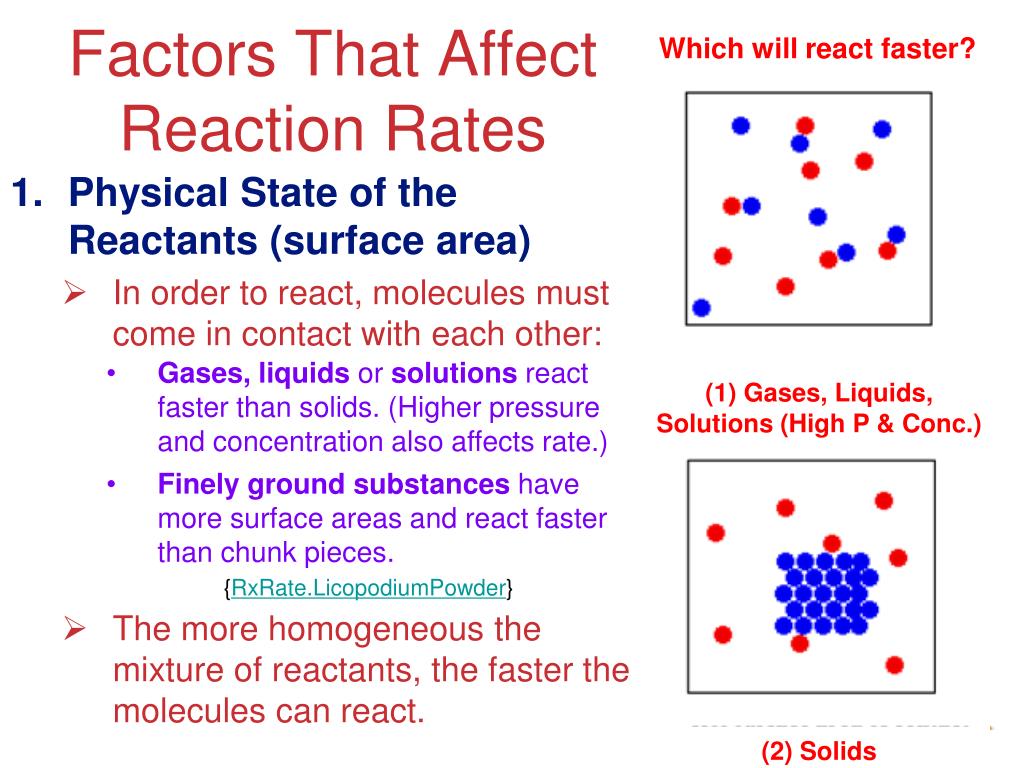
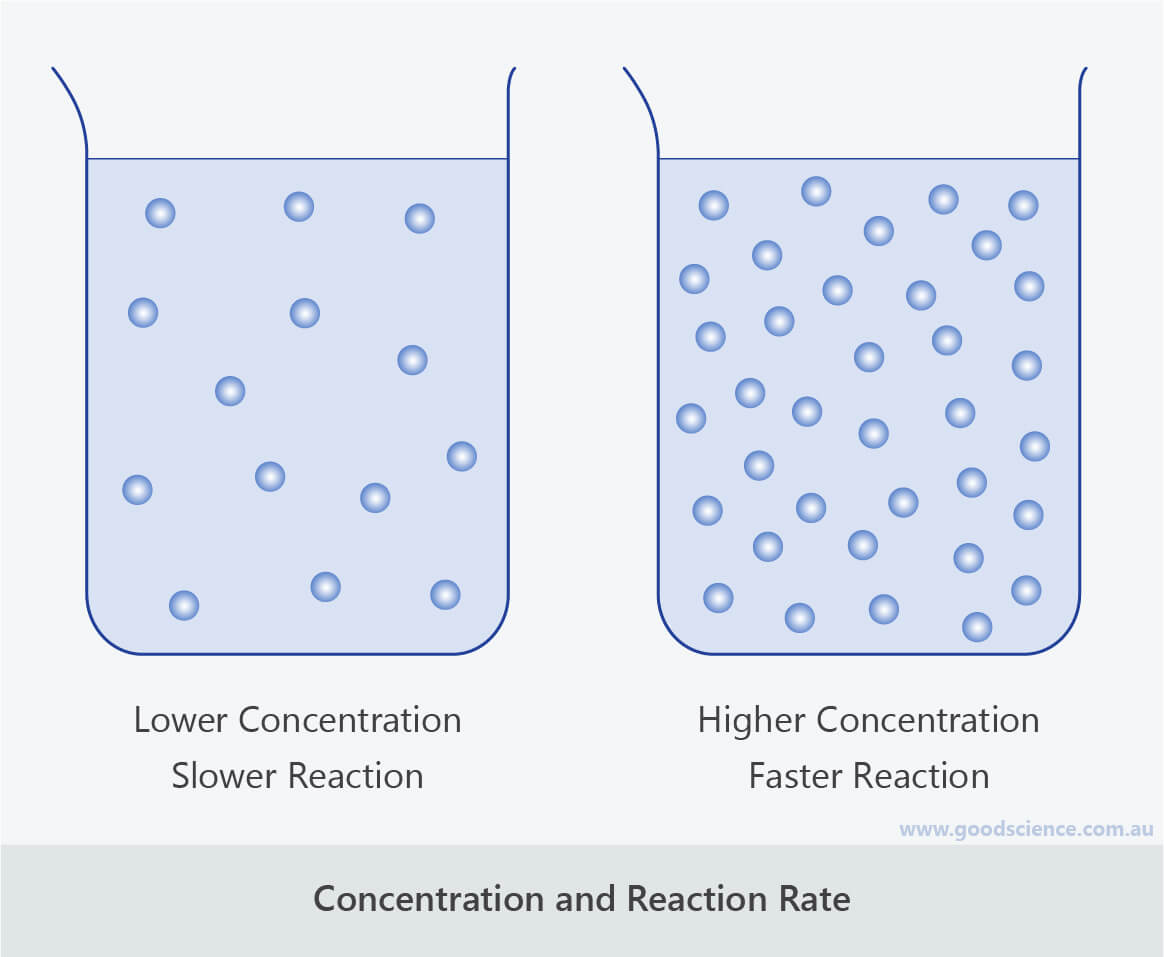
Increasing the concentration of either reactant increases the number of collisions, and therefore increases the number of successful collisions and the reaction rate. Six factors that affect rates of reactions. The reaction rate increases in the direction of less.
In general, anything that increases the number of collisions between particles will increase the reaction rate, and. Equation of rate of a chemical reaction. Three ways to increase the rate of a chemical reaction is by changing factors, such as concentration, temperature, and surface area.
Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the concentration of its. Rate of disappearance of r = decrease in concentration of.
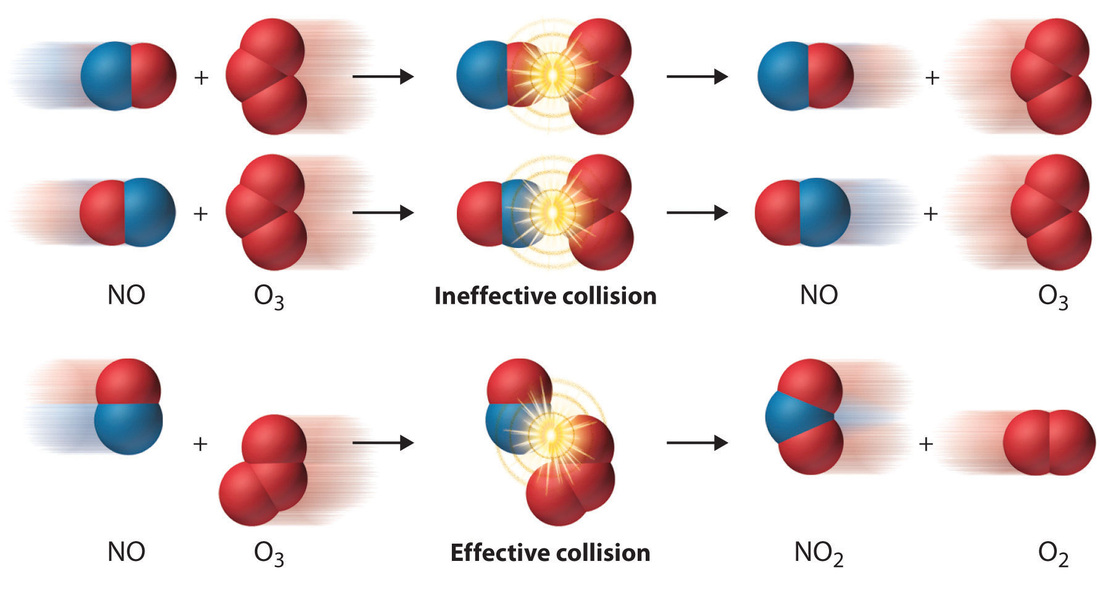
Chemical reactions can be viewed as mathematical functions that receive reaction conditions as input values and output reaction outcomes (e.g., product yield,. How do we know that? The rate of reaction is proportional to the number of collisions over time;
By monitoring the depletion of reactant over time, or Several factors can increase the rate of a chemical reaction. The primary reason for global extinction is a reduction in the heat release rate, and.
For the flow rate ratio of 0.5, this geometry offered a 10% increase in the mixing index and a decrease in the droplet diameter by 10% compared to the t junction. In general, increasing the concentration of a reactant in solution, increasing the surface area of a solid reactant, and increasing the temperature of the reaction system will all. The change of concentration in a system can generally be acquired in two ways: