Top Notch Info About How To Tell If Molecules Are Polar Or Nonpolar
Therefore, they are electrically charged.
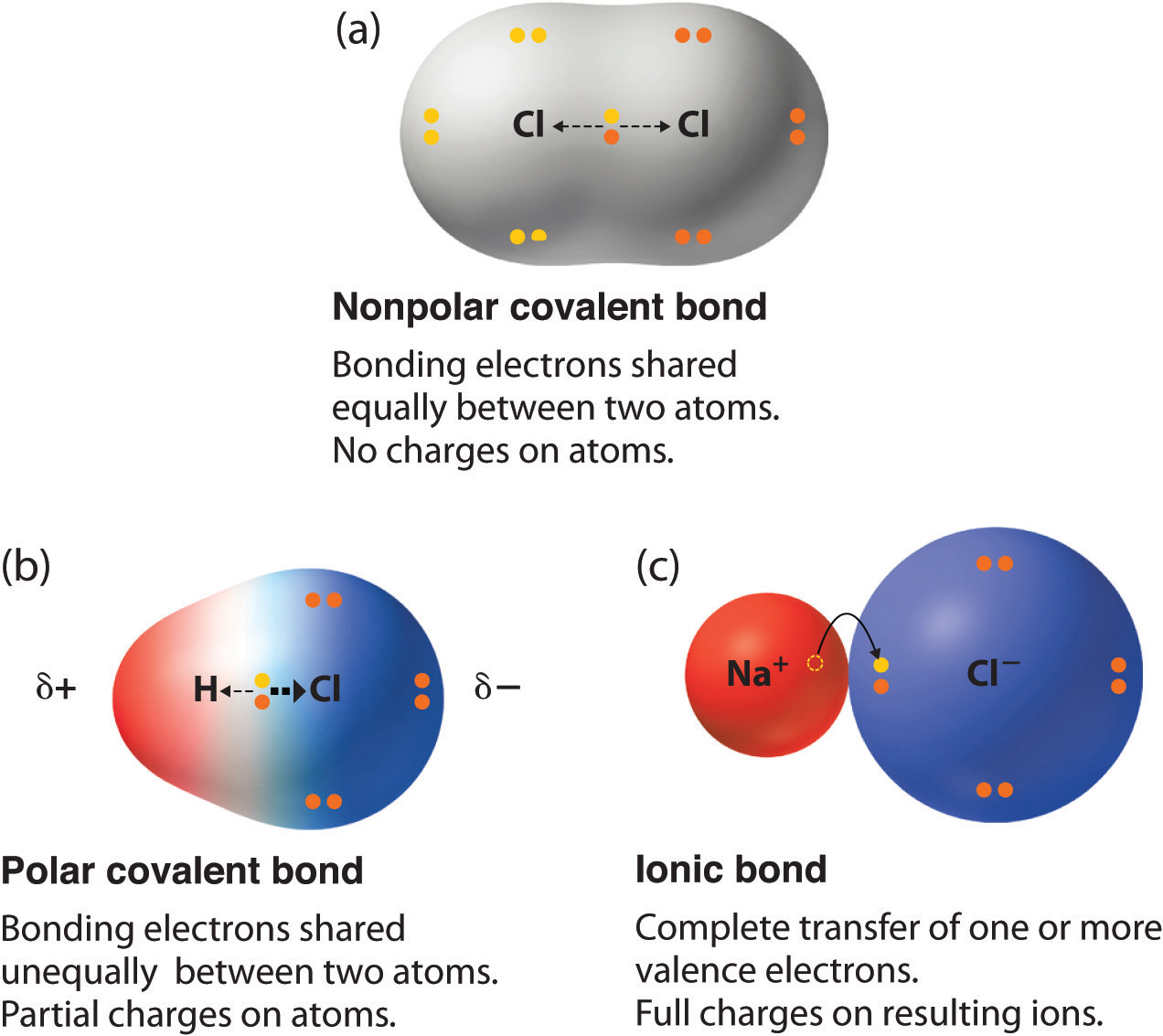
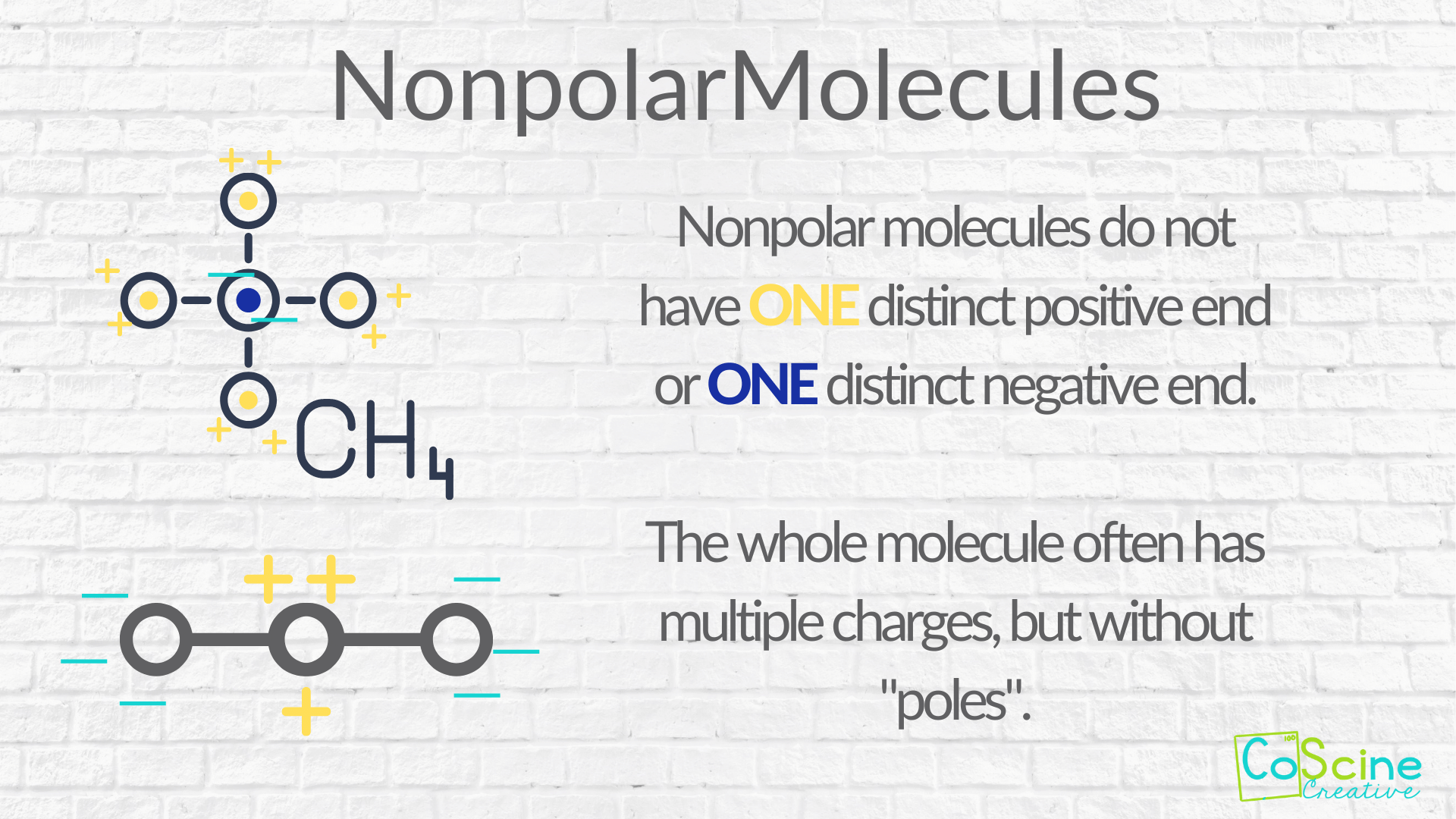
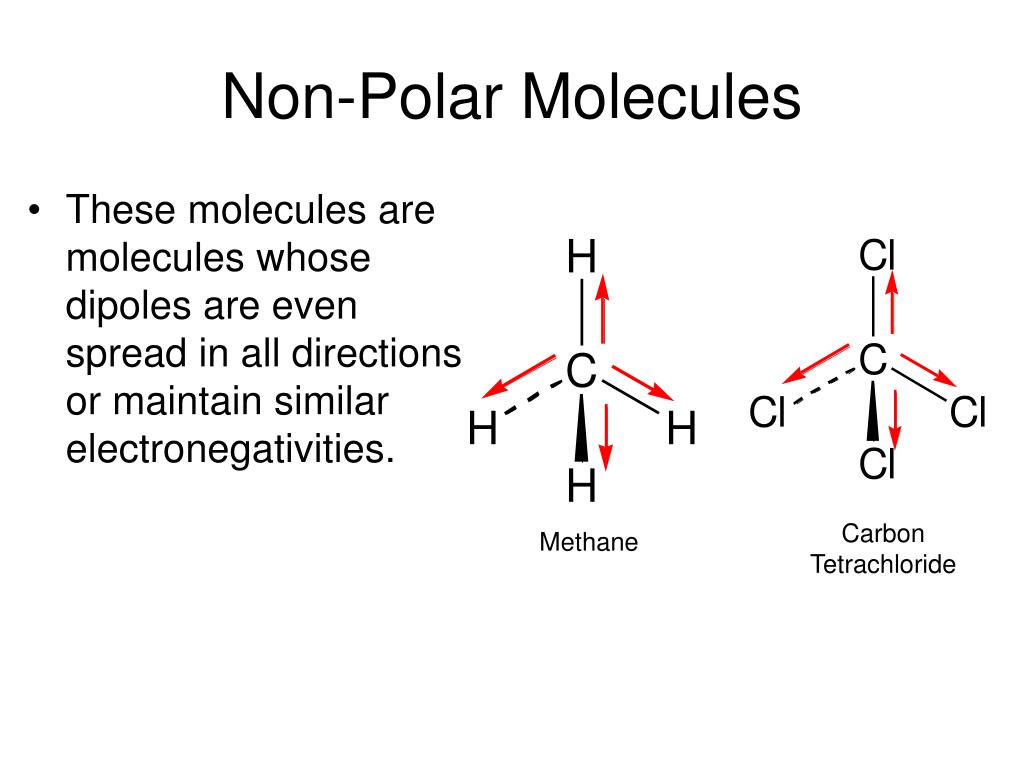
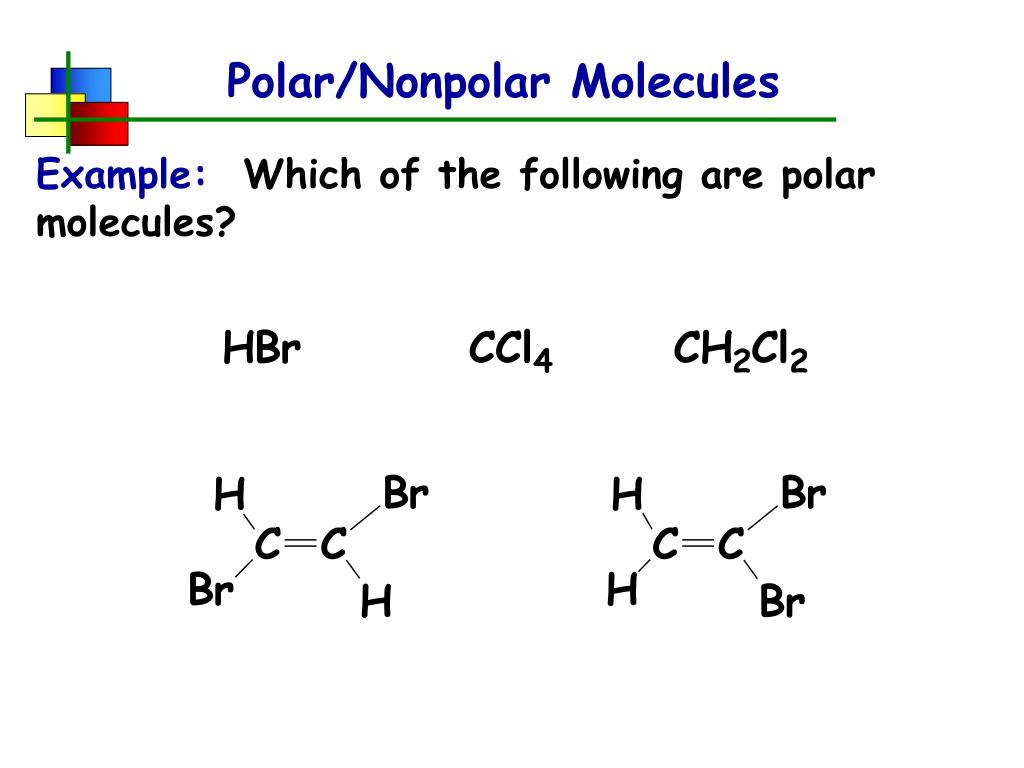
How to tell if molecules are polar or nonpolar. Result a molecule is polar if one part of it has a partial positive charge, and the other part has a partial negative charge. The atom that holds the electrons. How to draw lewis structures:
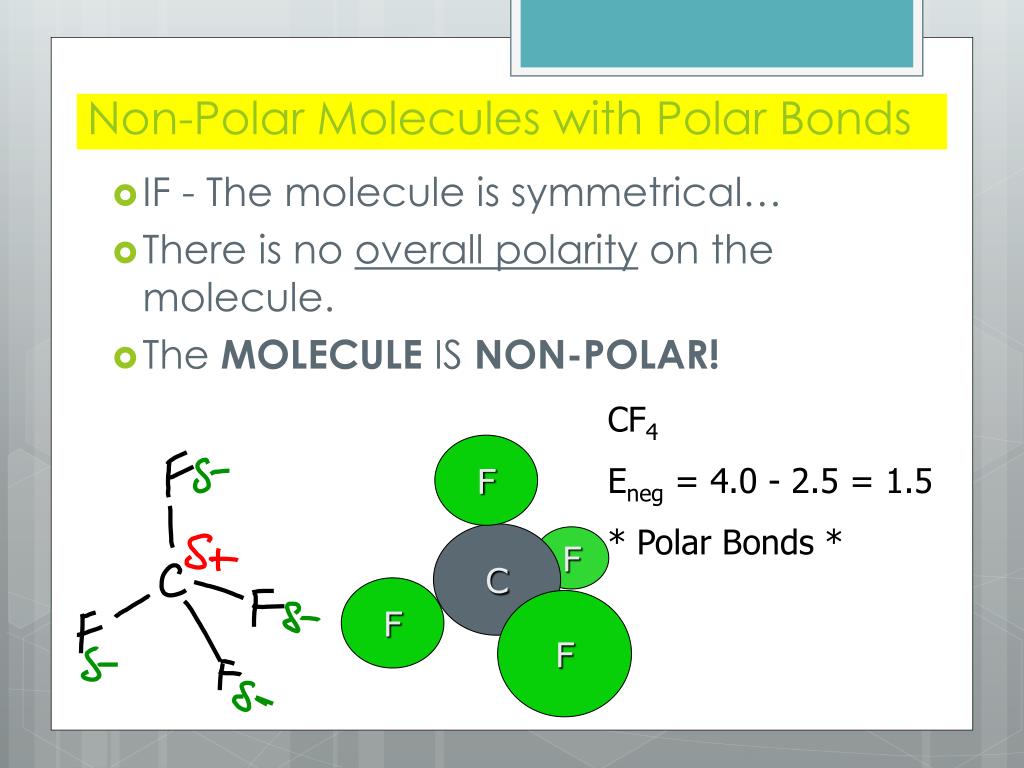
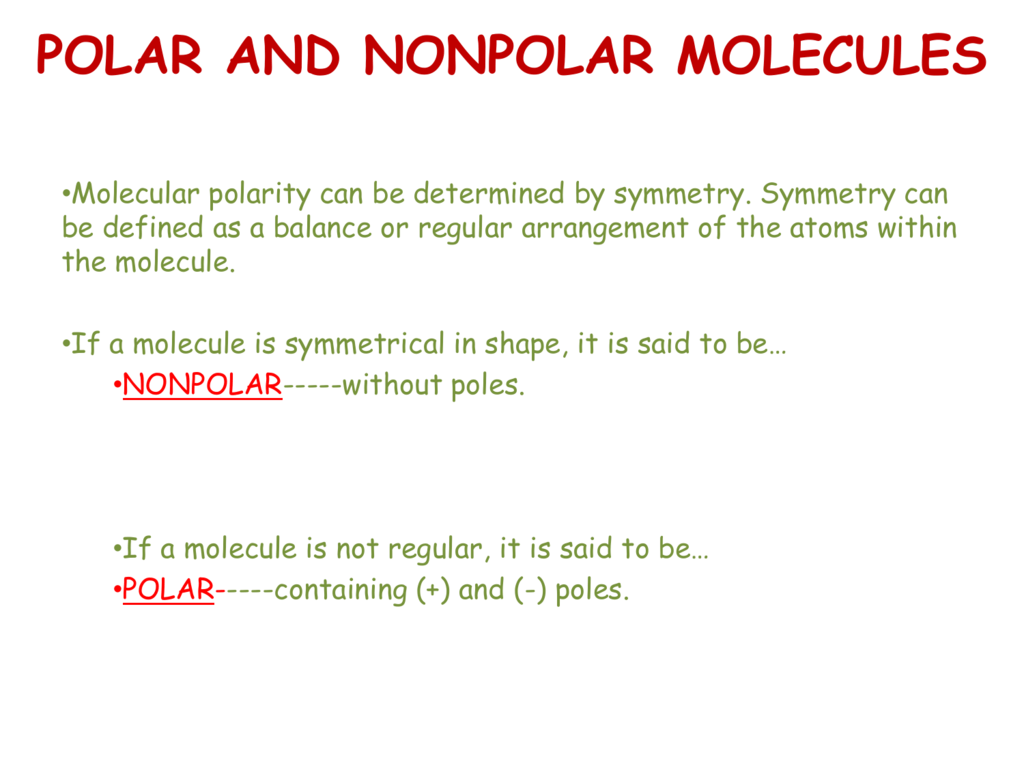
Result for molecules with more than two atoms, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. When in a bond, atoms can either share electrons (covalent) or give them up (ionic).
Result this video provides a fast way for you to determine if a molecule is polar or nonpolar. Updated on september 02, 2020. The linear molecular geometry and symmetrical arrangement of the.
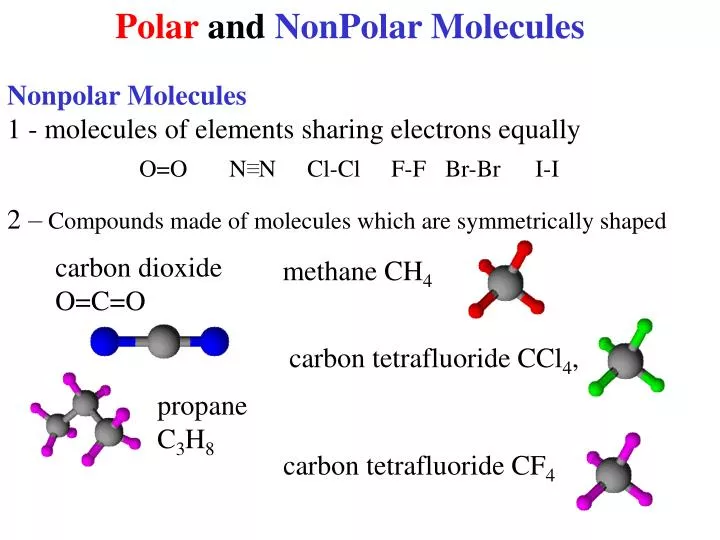
Some molecules are clearly polar or nonpolar, while others fall somewhere on. Bond polarity and molecular polarity are different (though related) concepts. The two main classes of molecules are polar molecules and nonpolar molecules.
4m views 10 years ago chemistry. Result nonpolar molecules. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and regions of partial positive charge.
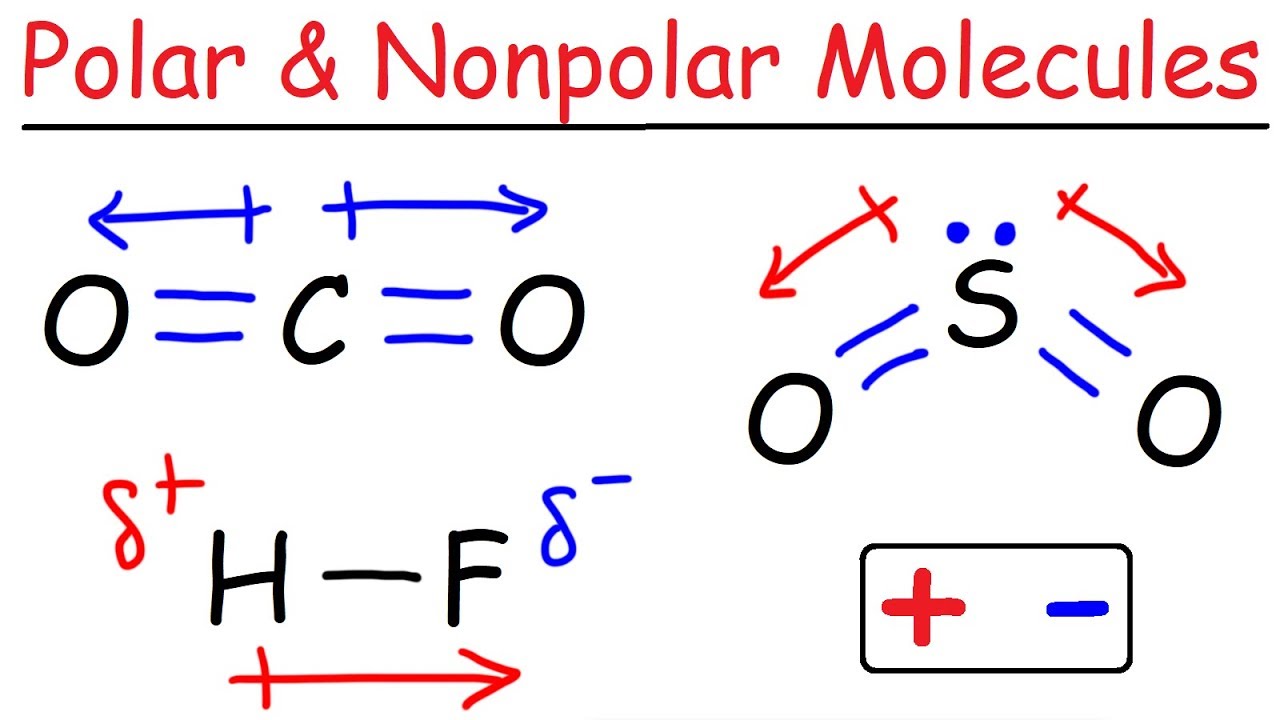
Result learn to determine if a molecule is polar or nonpolar based on the polarity between bonds and the molecular geometry (shape).we start with the polarity betwe. In contrast, water is polar because the oh bond moments do not cancel out. By varying the lengths of the arrows, the relative polarities.
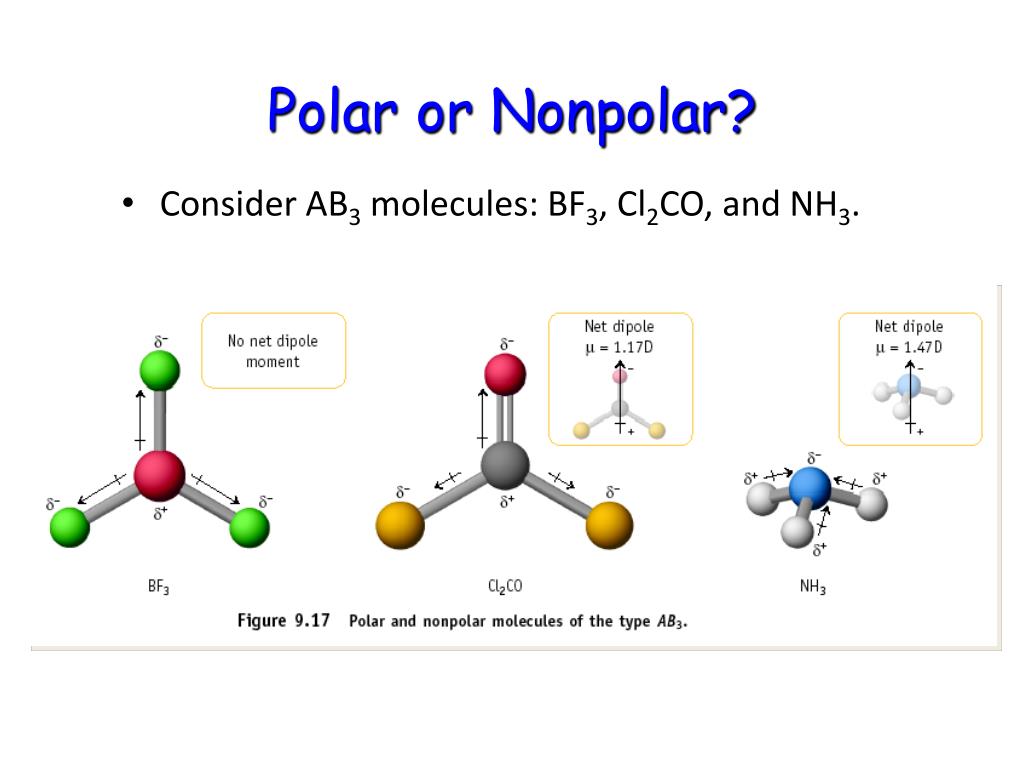
Like bonds, molecules can also be polar. Water, alcohol, sulphur dioxide, ammonia, ethanol, hydrogen sulfide, bent molecules (those with a significant bond angle) in general. In short the molecular dipole moment is the vector sum of the individual bonds dipole moments, so you have to know the geometry of the molecule to.
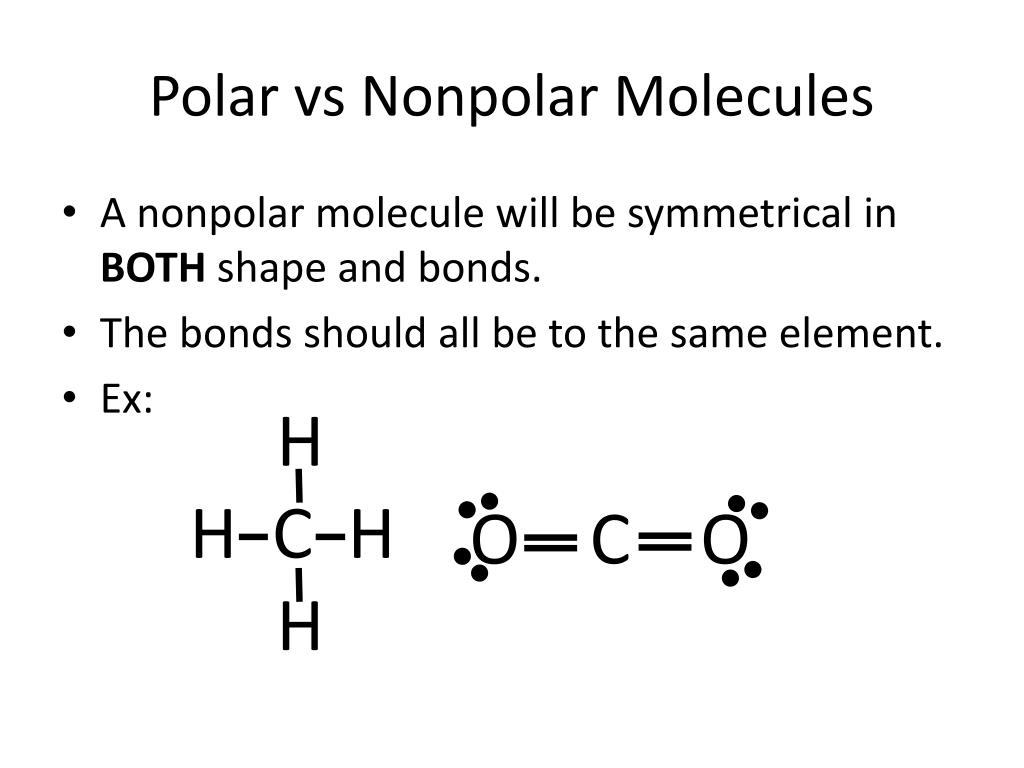
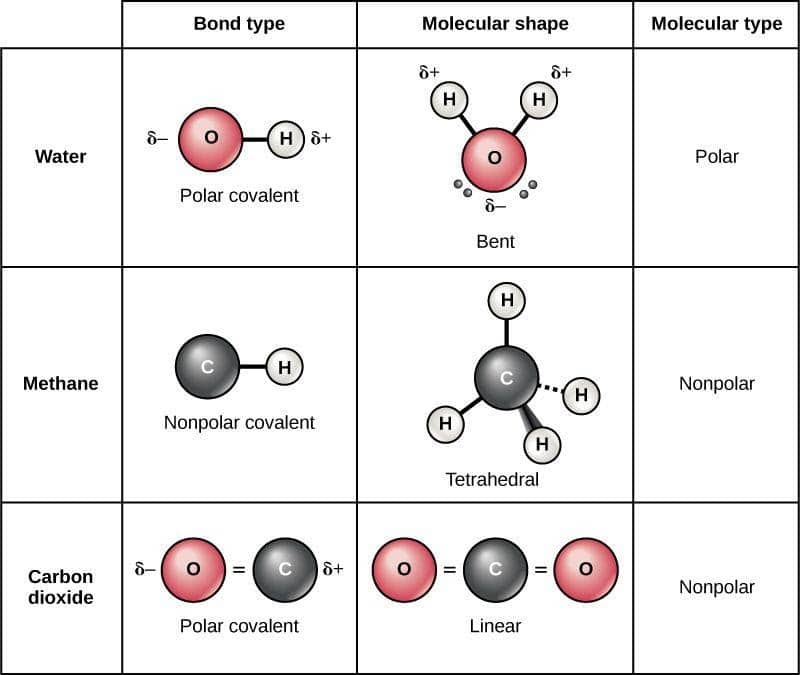
The figure below shows a comparison between carbon dioxide and water. Result polar molecules interact with other polar substances. Result each co bond has a dipole moment, but they point in opposite directions so that the net co2 molecule is nonpolar.
Result this chemistry video tutorial provides a basic introduction into polar and nonpolar molecules. Some examples of polar and nonpolar molecules based on molecular geometry. It provides examples so you can quickly distinguish nonpolar molecul.
These molecules have positive and negative charges on opposite ends.




:max_bytes(150000):strip_icc()/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)