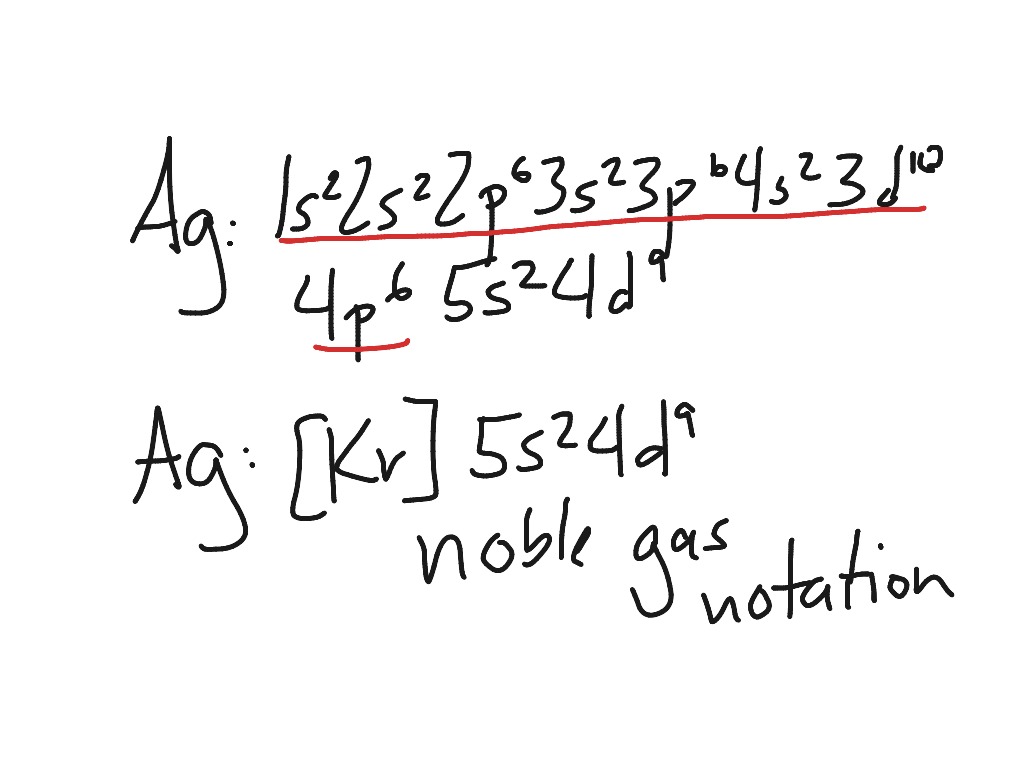Fun Info About How To Write Noble Gas Configuration

Find the previous noble gas.
How to write noble gas configuration. 329k views 8 years ago chemistry. Determine the atomic number of the element whose configuration is to be written. In this video, i'll explain how to write an electron configuration using noble gas notation.
Watch a video tutorial and see examples of noble gas configuration for the. Noble gas configuration | electronic structure of atoms | chemistry | khan academy. How to write noble gas configuration?
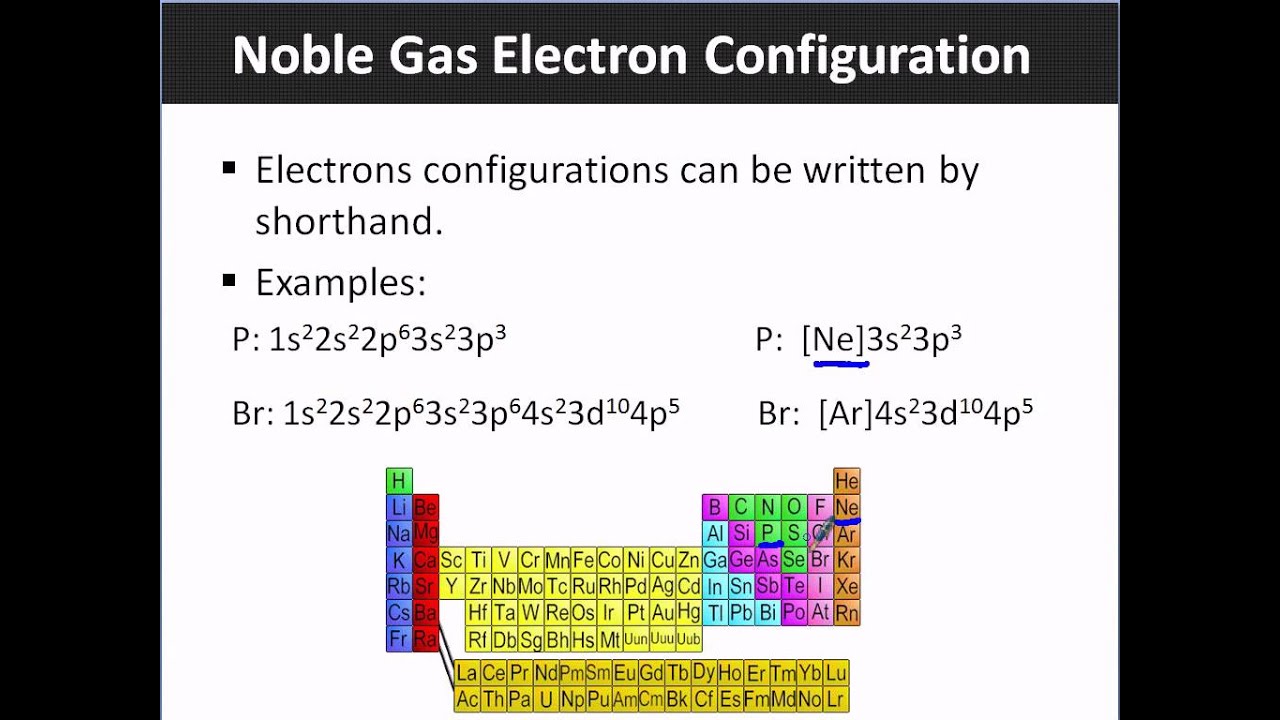
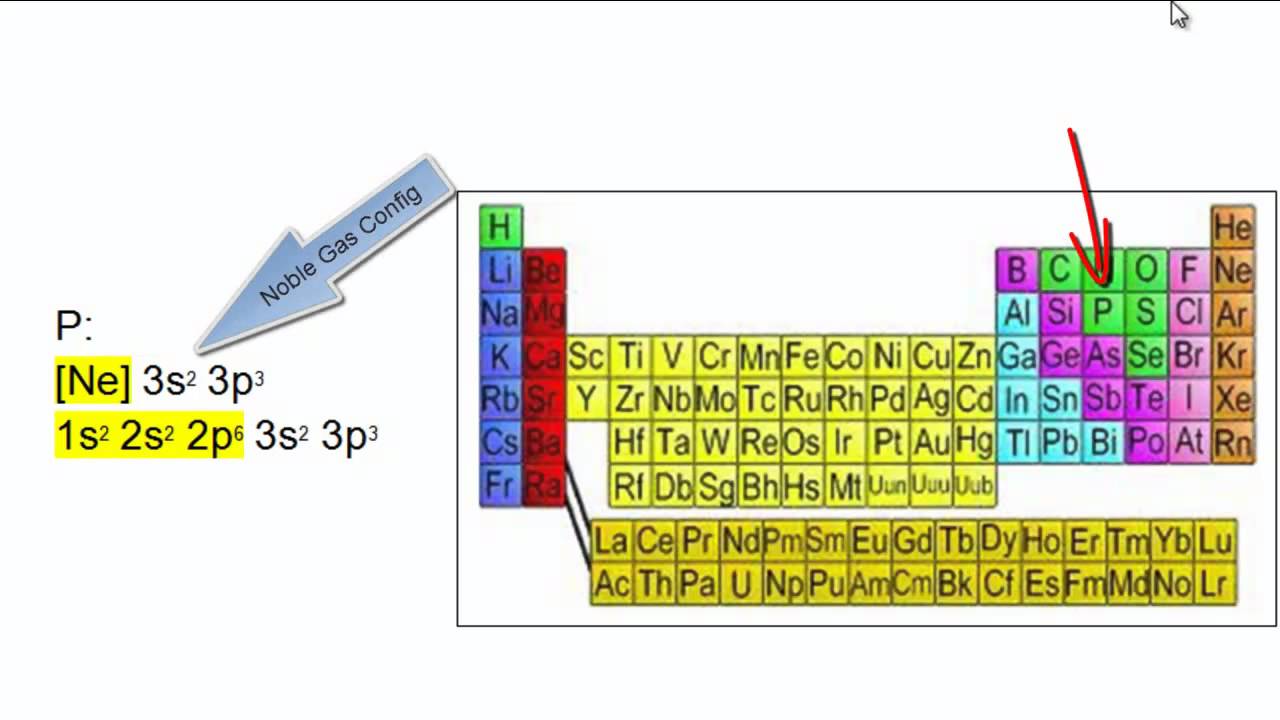
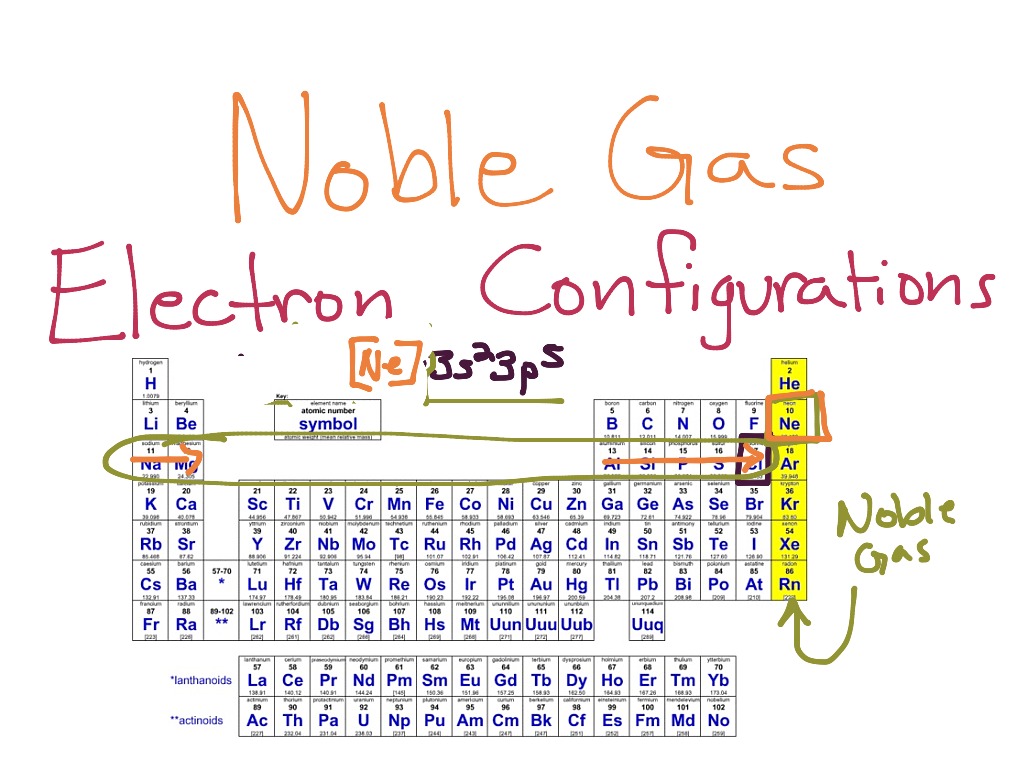
Learn how to write electron configurations for atoms and monatomic ions using noble gas configuration. So for sodium, we make the substitution of \(\left[ \ce{ne} \right]\) for the \(1s^2 2s^2 2p^6\) part. When writing noble gas electron configurations, start with the noble gas from the previous period.
The noble gas configuration system allows some shortening of the total electron configuration by using the symbol for the noble gas of the previous. Topics include electron configuration, diagonal rule, and noble. This video shows you how to write the ground state electron configuration using noble gas notation (abbreviation) for the elements fluorine, sulfur and cadmium.
194 views 6 months ago. A nobler gas core is one noble gas field symbol. For silver, z = 47.
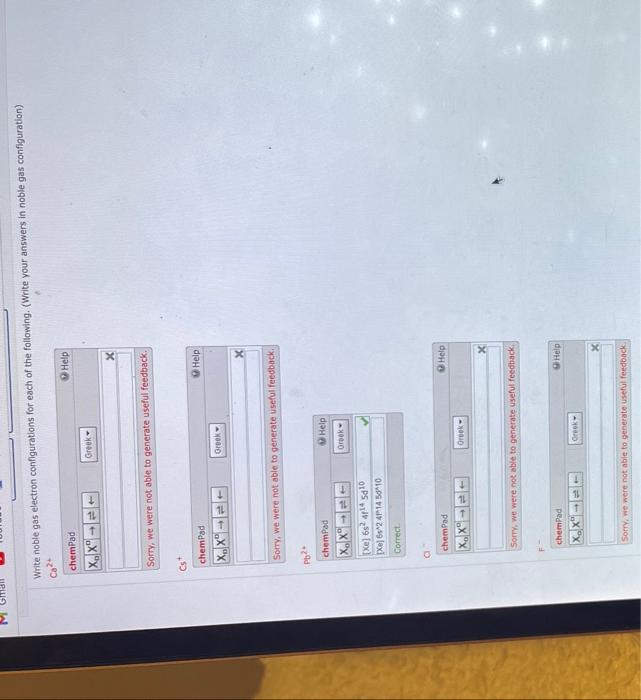
Writing electron configurations can be a long and daunting task, until you learn the shortcut! The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the table. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining.
This chemistry video explains how to write the electron configuration of an element using noble gas notation.speed of light, frequency, wavelength: Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core. The noble gas configuration is a way of summarizing the electrons of an atom that are.
Where is your periodic table? It is thus 11 protons removed from the last noble gas, which is krypton, z = 36. The symbol of the noble gas is placed in brackets,.
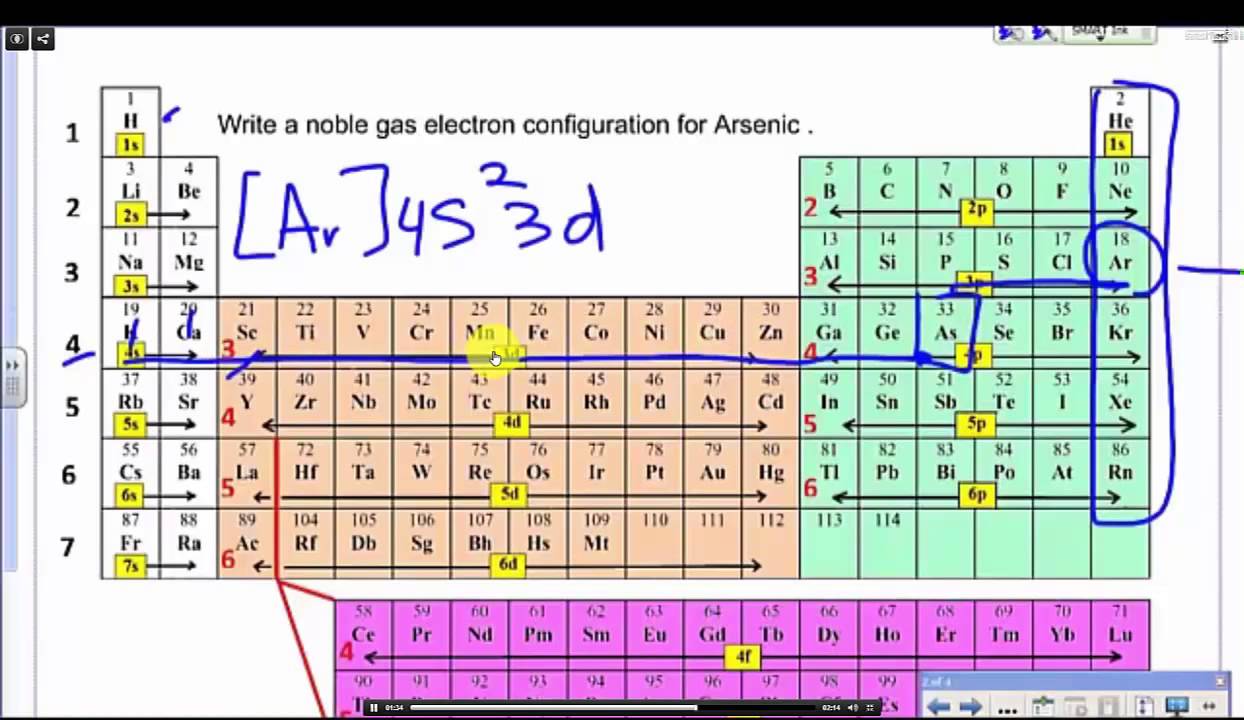
The trick is to start at the next smallest noble gas and then complete the electron. Type the noble gaseous configuration by writing the noble gas core, followed by the valence electrons. Learn how to write the noble gas configuration for any element using a simple formula.
A short cut method of writing configurations. 1s, 2s, 2p, 3s, 3p, 4s,.








![Abbreviated electron configuration calculator [Noble gas]](https://i0.wp.com/topblogtenz.com/wp-content/uploads/2022/04/how-to-write-shorthand-or-noble-gas-electron-configuration-min.png?resize=773%2C519&ssl=1)